Brewing Water Chemistry
Water is probably the most important ingredient in beer, after all, it accounts for 90-95% of beer's volume. Water in not just a carrier or dilutant for flavor and alcohol, trace compounds in water can either make beer delicious, or undrinkable. If you have ever had tap water with iron or sulfur or lots of chlorine in it, you know how nasty that can be, and if used untreated for making beer, the beer will also taste bad. But water is not just neutral or bad; trace compounds such as Calcium are important in chemical processes that occur when making beer, are necessary for the beer to turn out great. One doesn't need to have a PhD in chemistry to be able to make good beer (watch this video to learn how to make great beer) but a basic understanding of your water and what's in it will help you make great beer, consistently.
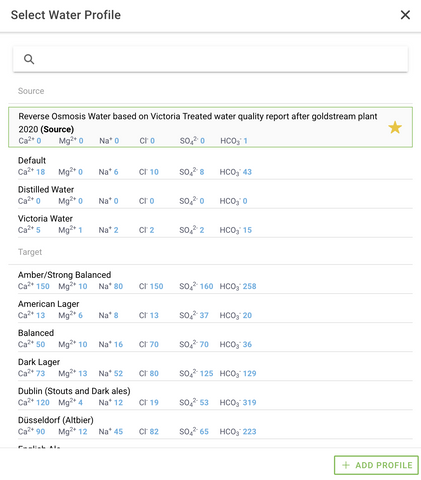
Entire books have been written on the subject of water chemistry in brewing, so there is a lot that can be learned, but for the beginner brewer, it is generally enough to know that tap water can be used if it is good quality; as a general rule if tap water tastes good, it can be used for brewing. However, if it contains a lot of minerals, metals or chlorine (anything that might contribute off-flavor), Reverse Osmosis (RO) or distilled water should be used (RO uses filter membranes and distilling uses evaporation to separate water from other molecules; neither process completely purify water, but both can remove almost all impurities). Softened water should not be used, as it often contributes too much sodium. (If one is wondering what is in their tap water, municipalities generally make their water reports available, and if using well water, companies can provide a detailed analysis for a fee.)
As mentioned above, some minerals, especially calcium, are necessary, so if using RO or distilled water, brewing minerals/salts need to be added back in. If a brewer enters the data from their local water report into Brewfather as a 'source water profile' and then selects a 'target water profile' for the style of beer they are brewing, Brewfather will automatically calculate how much of each brewing salt to add. Brewers can purchase the brewing salts from their local brew supply store.
We often think of 'salt' as something you have with dinner, but in chemistry, a 'salt' is a compound (two or more atoms) joined together by positive and negative charge; NaCl is just one salt among many—the sodium (symbol 'Na') has a positive charge, and the chloride (symbol 'Cl') has a negative charge; the two opposite charges attract each other and have no charge when joined. The following table outlines common brewers salts used for water adjustment.
|
Scientific Name |
Common Name | Notes |
| Calcium carbonate (CaCO3) | Chalk | Raises pH. Very limited solubility in water; should be added directly to the mash. Used for dark beers. |
| Calcium sulfate (CaSO4) | Gypsum | Lowers pH. Accentuates crispness and bitterness for hop-forward beers |
| Calcium chloride (CaCl2) | Lowers pH. Increases calcium as needed for soft water. | |
| Magnesium sulfate (MgSO4) | Epsom salt | Minimal effect on pH. Accentuates crispness and bitterness for hop-forward beers. |
| Sodium bicarbonate | Baking soda | Raises pH through increased alkalinity. Good addition for dark beers |
| Sodium chloride | Table salt | No effect on pH. Promotes saltiness |
| Magnesium chloride | Minimal effect on pH. Used to increase magnesium in deficient profiles without affecting sulfates |
The following table outlines the constituents in brewing salts, what they do, and their recommended usage in beer:
| Constituent | Use (ppm) | Notes |
| Calcium (Ca) | 50-150 | Instrumental to many yeast, enzyme, and protein reactions both in the mash and the boil. Promotes clarity, flavor and stability in the finished beer. (Level above 250ppm can inhibit fermentation.) |
| Magnesium (Mg) | 5-40 | Important for fermentation. Malt typically has enough so addition only important to add if making a recipe with high adjunct or refined sugar. (Level above 125ppm can have a laxative effect) |
| Sodium (Na) | 0-50 | Rounds out beer flavors and accentuates the sweetness of malt. (150-200ppm will taste salty and levels above 250ppm may taste harsh and sour) |
| Chloride (Cl) | 0-100 | Helps accentuate malt sweetness and beer fullness. (Levels above 300ppm negatively affect yeast health) |
| Sulfate (SO4) | 0-250 | Accentuates hop bitterness, making the bitterness seem drier and more crisp. (Levels above 400 make the beer astringent and unpleasant) |
| Carbonate (HCO3) | 0-200 | Bicarbonate is alkaline and raises the pH (makes it less acidic). High levels are problematic for mashing and promote scale when combined with calcium and magnesium |
Note: these two tables are sourced from Mastering Brewing Science by Farber and Barth, and Water: A Comprehensive Guide for Brewers by Palmer and Kaminski
Instruction video on how to make amazing beer
Benefits of the BIAC complete microbrewery system
BIAC complete brewery equipment product page